There is an increasing need to build research capacity to address the emerging threat of Lyme disease (LD) in Canada. We are establishing the Canadian Lyme Disease Research Network (CLyDRN) to provide national capacity to conduct integrated, multidisciplinary, evaluative, and discovery research. CLyDRN will support, link, and coordinate research activities conducted in academia, public health, health care, and industry. CLyDRN’s research planning and activities will be conducted in collaboration with stakeholders including patients, caregivers and family members, community leaders and members of the general public (citizens), health care providers (HCPs), and public health professionals (PHPs). The goal of this research network is to improve prevention, diagnosis, and treatment of LD in Canada.

About the Network
Mission
This vision will be accomplished through a multi-centric, collaborative and team-based approach. The research group will make use of existing resources, and expertise from various academic and public health institutions as well as professional associations and commercial enterprises.
Values
Innovation and Excellence. The research network would be the first group of its kind at a Canadian university and would promote new and meaningful research
Collaboration. The goal of the research group would be to bring experts from various disciplines together, including clinicians, microbiologists, mathematicians, biologists, and public health professionals amongst others to form a multidisciplinary team with a common interest in LD and other tick-borne illnesses
Relevance. The research group would explore areas significant to Canadians and their needs, given that tick-borne illnesses are becoming an increasingly pressing issue
Enabled by the network, our vision of reducing the impact of LD through addressing knowledge gaps in Canada will be accomplished through this multidisciplinary approach which will mobilize and build LD-specific capacity and training opportunities, improve clinical science and practice, provide best evidence in testing and patient care, improve patient and population prevention outcomes and inform evidence-based policy.
Foundational Resources will support and guide all network research objectives and activities defined within the network’s four Research Pillars.
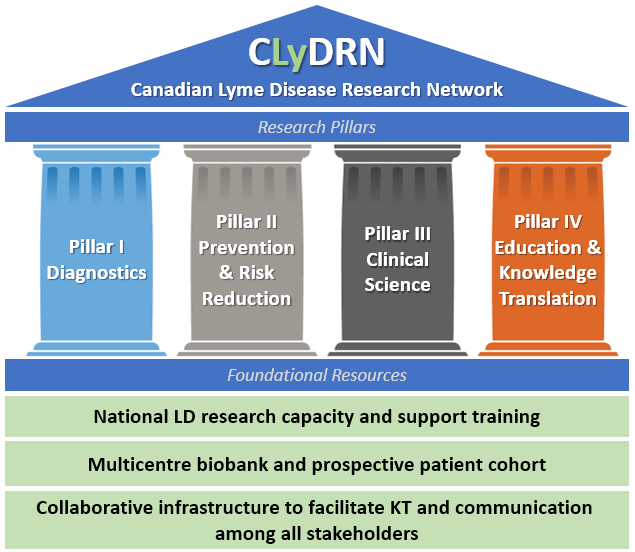
Research Pillars
Pillar I. Diagnostics. Using biobank samples and associated clinical cohort data, we will fund projects through a seed grant program that will develop and evaluate novel diagnostics platforms and methods to: 1) improve diagnostic test sensitivity, accuracy and/or speed in early LD and in non-endemic regions where limitations in positive predictive power of current testing are problematic; and 2) identify novel non-antibody-based biomarkers to improve sensitivity of early testing, monitor disease progression, resolution and treatment outcomes, distinguish patients with different disease symptoms, identify patients at risk of experiencing post-antibiotic treatment LD symptoms (PTLDS), and identify biomarkers of PTLDS that may lead to improved understanding and treatment of these symptoms. We will evaluate the ability of these and current two-tier serological tests to detect and possibly distinguish infection with different Lyme disease Borrelia strains present in Canada.
Pillar II. Prevention and Risk Reduction. We will support research to identify and evaluate preventive measures, interventions, and/or population health approaches to decrease risk of occurrence of LD, including a population cohort-linked multi-site longitudinal study to investigate environmental, social and epidemiological LD risk determinants. Additionally, seed grants will allow us to build regional models and maps to predict fine-scale risk and investigate novel risk reduction strategies. These activities will provide a quantitative framework to identify, develop and evaluate novel approaches for measuring and reducing LD risk.
Pillar III. Clinical Science & Health Services Research. We will support research to determine the natural history of LD and identify and clinical (e.g., comorbidities) and other (e.g., bacterial strains, environmental, co-infections) risk factors for LD infection and progression (manifestations, sequelae). We will determine the impact of clinical management (diagnosis, treatment) on disease outcomes of Borrelia infection. We will determine the health and economic burden of LD from a wide range of perspectives. Understanding the burden of disease, natural history of disease, clinical management and risk factors will allow us to identify and assess integrated intervention strategies encompassing targeted and universal environmental (e.g., risk reduction strategies) and clinical interventions.
Pillar IV. Patient & Community Engagement, Training and Knowledge Translation. We will conduct research, in collaboration with community and patient stakeholders, on LD assumptions, perspectives, concerns, and goals held by the general public, HCPs, and patients to establish a basis for developing effective approaches to knowledge translation, training, and information dissemination. The goal of this work will be to enhance communication and collaboration between stakeholders for improved LD diagnosis, treatment, and the development and adoption of evolving best practices.
EVENTS
TickNet Canada Scientific Symposium:
https://event.fourwaves.com/ticknet/pages

Lyme Disease and Tick-borne Diseases Awareness Event (May 1-31, 2023)
May is Lyme Disease Awareness Month…Go GREEN for Lyme!
To help raise awareness for Lyme disease, we are holding a Virtual Lyme Disease and Tick-borne Diseases Awareness Event throughout the month of May (May 1st-31st, 2023).
Sign up for our live virtual presentations on Lyme disease and Tick-borne diseases topics. Please send your name and email to Clydrn@gmail.com and a ZOOM meeting link will be provided to allow you to view all virtual presentations scheduled throughout the month.
Program Agenda: CLyDRN Virtual Lyme Disease Awareness Event May 2023 Program REVISED FINAL (General Distribution)
Help take a bite out of Lyme disease…Show your support for Lyme disease by wearing green in May.
Take part in our challenge: wear green, take a photo and share it with us to help spread awareness of Lyme disease. You can even send in photos or drawings that express Lyme Disease Awareness Month…be creative. All photos and drawings submitted will automatically be entered into a draw for 1 of 4 $25 Starbucks gift cards. Draw will occur on May 31st, 2023. Please send you photos to Clydrn@gmail.com along with your name and email.
2022 Annual General Meeting (AGM) (November 2, 2022)
We are holding our Virtual Annual General Meeting on November 2, 2022 (10:00 AM-4:30 PM EST).
Sign up for our live AGM. Please send your name and email to Clydrn@gmail.com and a ZOOM meeting link will be provided to allow you to view all pillar and committee presentations scheduled throughout the afternoon.
Program Agenda: CLyDRN Virtual Fall 2022 AGM Program FINAL (November 2, 2022)